Many articles about antibodies refer to the term “humanization”.
But what does humanization mean?
What technical approaches are used to carry out such a process? What are the potential applications?
Let’s take a look and go back to the very beginning of the story…
In terms of immunogenicity, when foreign material is injected into a dedicated host, the latter triggers an immune response against the former. Consequently, in the ’80s, most therapeutic monoclonal antibodies of rodent origin faced the difficult challenge of the generation of immune complex in patients (a.k.a. the human anti-mouse antibody response or HAMA).
Biotech companies made the choice to replace step-by-step animal components by human ones in order to tackle these safety concerns.
The concept of humanization was born.
To humanize an antibody is to introduce as much human content as possible to reduce the risk of immunogenicity linked to non-human regions while retaining enough non-human content to maintain the original binding activity of the parent antibody.
It seems easy on the paper, huh? But requires fine-tuning in practice….
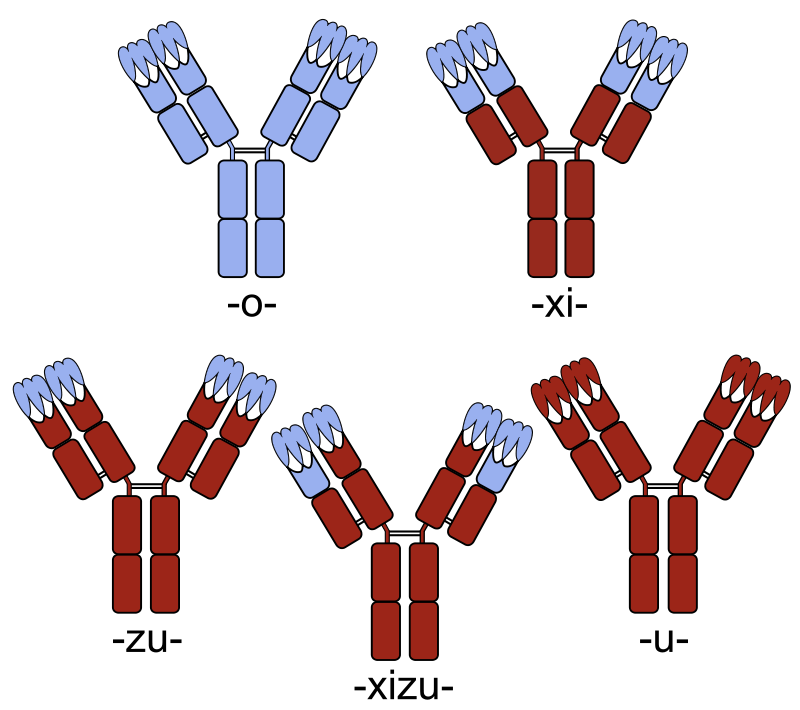
The different terminologies of antibodies based on nomenclature Fully mouse -o- (e.g. OKT3, muromonab-CD3),
chimerized –xi (e.g. anti-CD23 lumiliximab), humanized -zu- (e.g. anti-IgE omalizumab),
and fully human –u- (e.g. anti-CD20 ofatumumab).
The first upgrade was chimerization, keeping a murine variable domain on a human scaffold.

To produce chimeric antibodies, rodent hybridoma are produced by the classic hybridoma method. The gene coding for the rodent variable region of heavy and light chains are isolated and amplified by polymerase chain reaction (PCR).
Constant-region genes of human heavy and light chains are also amplified. After insertion of human and murine genes into a plasmid, chimeric antibodies are produced as a recombinant entity.
But when chimerization is not sufficient enough in terms of safety, you need to take it one step further and go for humanization.
The grafting of mouse complementarity-determining residues (CDRs) onto human acceptor Ab frameworks is the most used and mastered method to get a humanized antibody. This process is performed on the same basis as chimerization, starting from cDNA hybridoma sequence and testing the antigen-binding potency of the humanized entity via several rounds of iterative optimization.
And this is typically what we offer to our customers.
But surprisingly enough, not only to biotech and pharma companies!
Increased demand is emerging from another field, that of the In-Vitro Diagnostics (IVD) market, where kits are developed to measure markers in human plasma matrix, and where it seems of interest to have a human scaffold as antibody reference.

